Further to the cyberattack on EMA last year, some of the unlawfully accessed documents including email correspondence have been made public through the Internet and were subsequently picked up by some media outlets. This was the subject of a public update from the Agency on 15 January. A closer investigation of the published material has revealed that not all of the documents were published in their integral, original form and may have been taken out of context. Whilst individual emails are authentic, data from different users were selected and aggregated, screenshots from multiple folders and mailboxes have been created and additional titles were added by the perpetrators in a way which could undermine trust in vaccines.
In the EU, several measures are in place to ensure the continued high quality of vaccines over their lifecycle. Vaccines used in public health immunisation programmes are subject to stringent testing and quality control measures and must meet rigorous specifications approved by EMA. Additionally, an official medicines control laboratory of a Member State performs an independent control for each batch of vaccine.
The Agency continues to fully support the criminal investigation led by law enforcement authorities, in cooperation with other entities.
The Agency and the European medicines regulatory network remain fully functional and timelines related to the evaluation and approval of COVID-19 medicines and vaccines are not affected.
EMA will continue to provide information in due course, to the extent possible, given its duty towards the ongoing investigation.
European Medicines Agency (EMA)
Fachartikel

Strategien für eine fortgeschrittene digitale Hygiene

Mit LogRhythm 7.16 können Sie das Dashboard-Rauschen reduzieren und Log-Quellen leicht zurückziehen

Wie man RMM-Software mit einer Firewall absichert

Red Sifts vierteljährliche Produktveröffentlichung vom Frühjahr 2024
Konvergiert vs. Einheitlich: Was ist der Unterschied?
Studien

Studie zu PKI und Post-Quanten-Kryptographie verdeutlicht wachsenden Bedarf an digitalem Vertrauen bei DACH-Organisationen

Zunahme von „Evasive Malware“ verstärkt Bedrohungswelle

Neuer Report bestätigt: Die Zukunft KI-gestützter Content Creation ist längst Gegenwart

Neue Erkenntnisse: Trend-Report zu Bankbetrug und Finanzdelikten in Europa veröffentlicht

Studie: Rasantes API-Wachstum schafft Cybersicherheitsrisiken für Unternehmen
Whitepaper
Unter4Ohren

Datenklassifizierung: Sicherheit, Konformität und Kontrolle

Die Rolle der KI in der IT-Sicherheit
CrowdStrike Global Threat Report 2024 – Einblicke in die aktuelle Bedrohungslandschaft
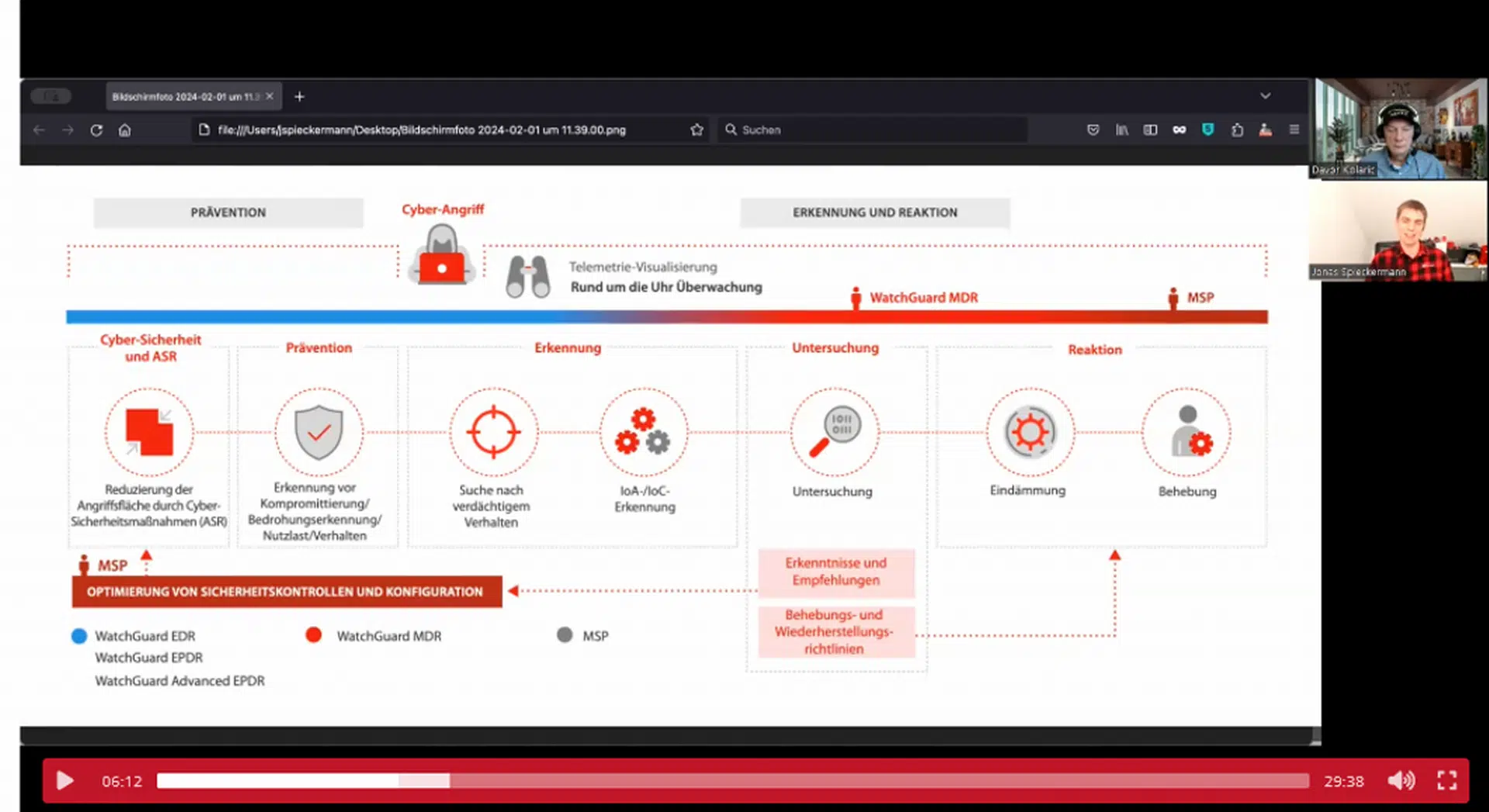
WatchGuard Managed Detection & Response – Erkennung und Reaktion rund um die Uhr ohne Mehraufwand